The content of this website is intended for United States audiences only.
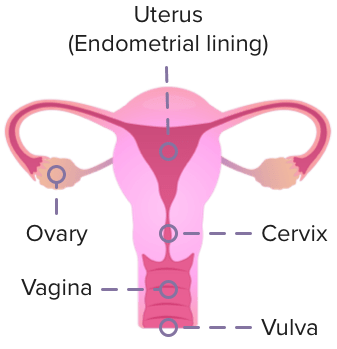
Gynecologic cancer is a condition where the cells in a woman's reproductive system start to grow uncontrollably.
This can happen in any of the 5 main areas of the female reproductive system:
Endometrial cancer is the most common type of gynecologic cancer in the United States. The number of cases has been rising steadily over the last 10 years. Each year, there was about a 1% increase in endometrial cancers in White women and a 2% to 3% increase among women of all other racial and ethnic groups.
Endometrial cancer is a condition where the cells in the lining of the uterus grow more than they should. The uterus is the part of a woman's body, located in the lower belly area between the hips. Symptoms of endometrial cancer may include abnormal vaginal bleeding or pelvic pain.


Ovarian cancer is commonly diagnosed in women between 55 and 64 years old. White, non-Hispanic American Indian, and Alaska Native women are at a higher risk compared to Black, non-Hispanic Asian/Pacific Islander, and Hispanic women.
Ovarian cancer is a condition where cells in the ovaries, fallopian tubes, or peritoneum start to grow more than they should. Unlike cervical cancer, the cause of most ovarian cancers is unknown. Symptoms of ovarian cancer may include bloating, pain in the pelvic area (between the stomach and thighs), trouble eating or feeling full quickly, or urinary symptoms such as constant need to urinate or frequent urination.

Cervical cancer is commonly diagnosed in women between 35 and 44 years of age. Black and Native American women have a 65% higher risk of death from cervical cancer compared to White women. Despite a 1.7% annual increase in cases for women aged 30-44 years from years 2012-2019, the 20-24 years age group shows promise with an 11% annual decrease, likely due to the HPV vaccination.
Cervical cancer is a condition where cells in the cervix start to grow more than they should. The main cause is a long-lasting infection with human papillomavirus (HPV). Symptoms of cervical cancer may include abnormal vaginal bleeding, pain in the pelvic area (between the stomach and thighs), or an unusual discharge from the vagina.

Patients can find additional help and educational information from the following organizations:
We're working to discover, develop and deliver innovative therapeutics for people with life-threatening diseases.
We're working to discover, develop and deliver innovative therapeutics for people with life-threatening diseases.