The content of this website is intended for United States audiences only.
IBD involves long-term inflammation in your digestive system. About 1 out of 100 Americans are diagnosed with IBD each year. The 2 most common types of IBD are ulcerative colitis and Crohn’s disease.
Ulcerative colitis is a long-term condition that involves inflammation in the innermost layer of the lining of the colon (the longest part of the large intestine). It typically starts with small sores or ulcers in the rectum, which spread upwards through the colon. Symptoms may include diarrhea, bloody stools, abdominal cramping, and weight loss.
Most people are diagnosed with ulcerative colitis in their mid-30s. It occurs in men and women equally, and affects people of any racial or ethnic group.
The exact cause of ulcerative colitis remains unclear. However, it does tend to occur more often in people who have a family history of inflammatory bowel disease.

Ulcerative colitis is commonly treated with medications that can decrease inflammation, such as mesalamine or corticosteroids.
Moderate to severe cases that require long-term treatment are typically treated with immunosuppressants or biologics. These treatments are frequently administered through an IV or injection. However, some people may prefer the convenience of an oral treatment.

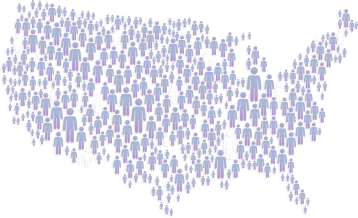
In the United States, approximately
have ulcerative colitis
Crohn’s disease is a long-term condition that also involves inflammation, but it can affect any part of the gastrointestinal tract. It most commonly impacts the small intestine, and often spreads into the deeper layers of the intestinal lining. Symptoms may include abdominal pain, persistent diarrhea, fatigue, weight loss, and loss of appetite.
Crohn’s disease can happen at any age but usually starts when people are between 15 and 35 years old. It affects men and women equally and people from all ethnic backgrounds.
The cause of Crohn’s disease is not fully understood. But hereditary, genetic, and environmental factors may play a role, according to recent research.

We're working to discover, develop and deliver innovative therapeutics for people with life-threatening diseases.
We're working to discover, develop and deliver innovative therapeutics for people with life-threatening diseases.